Using Clear Blue Easy Fertility Monitor to Avoid
Practice
Personal fertility monitors for contraception
CMAJ January 11, 2011 183 (1) 73-76; DOI: https://doi.org/10.1503/cmaj.090195

In 1990, Austrian chemist Carl Djerassi, who discovered the synthetic progestin used in the first birth control pill, expressed concern about existing contraceptive methods and predicted fertility awareness and family planning through biochemical measurement of endogenous hormones at home.1 Today, multiple fertility monitors are available that provide couples with accurate biochemical measurement of the fertile window to plan or avoid pregnancy, without potential side effects associated with other contraceptive methods. In this article, we review two new hormone-based personal fertility monitors that can be used for contraception.
Fertility monitoring involves biochemical identification of the fertile period and avoidance of intercourse, or use of other precautions, during this brief stage. The current understanding of the menstrual cycle is that an ovum in women of reproductive age is released once per cycle and is typically fertilizable for 12–24 hours, whereas sperm are typically viable for three to five days. In a series of studies by the World Health Organization in the 1970s, measurable changes in serum estradiol levels were found to signal the onset of potential fertility, and surges in luteinizing hormone levels were confirmed as the best predictor of impending ovulation.2 Subsequently, rising urine levels of estrone-3-glucuronide were found to correlate with rising serum estradiol levels and potential fertility,3 whereas surges in urine levels of luteinizing hormone accurately predicted impending ovulation. These findings showed that serum samples were no longer needed.
How do fertility monitors work?
The measurement of estrone-3-glucuronide and luteinizing hormone levels in urine to identify the fertile period was the premise in the development of two family-planning devices that first came to market about 10 years ago. The Persona monitor (Swiss Precision Diagnostics GmbH, Switzerland) was marketed for contraception; although currently available only in Europe, the device may be purchased in North America via the Internet. The second device was marketed for assistance in achieving pregnancy; it is distributed as Clearblue Easy and Clearplan Easy in North America and Europe (Swiss Precision Diagnostics GmbH) and as Clearview Primera in Japan (Mitsui Pharmaceuticals Inc., Japan). Using the same technology to indicate fertility, the Persona and Clearblue Easy fertility monitors use a urine-based test strip with antibodies to estrone-3-glucuronide and luteinizing hormone that detect elevated concentrations of these compounds.
Differences exist between the user instructions and sensitivity of these two fertility monitors. The Clearblue Easy monitor (Figure 1A) displays a "low," "high," or "peak" fertility reading, where "high" refers to an elevated urine estrone-3-glu-curonide level and "peak" refers to the surge in urine luteinizing hormone level. Given that the Clearblue Easy monitor is designed as a tool to achieve pregnancy, its use to avoid conception should be considered "off label." As a contraceptive device, Persona (Figure 1B) displays a green light during the infertile phase and a red light during the fertile phase, during which it also displays a symbol to indicate the surge in luteinizing hormone and impending ovulation. The Persona monitor has a lower threshold for detection of estrone-3-glucuronide and therefore indicates a slightly longer window of possible fertility before ovulation than the Clearblue Easy fertility monitor does. Accordingly, the Persona monitor may be more reliable for the minority of women who have short fertility phases.
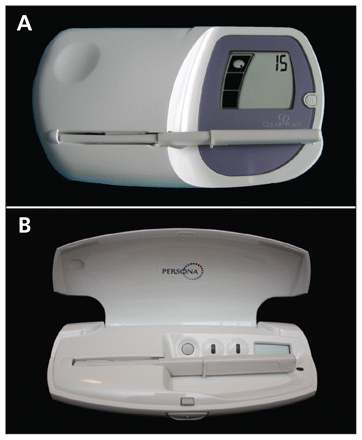
- Download figure
- Open in new tab
- Download powerpoint
Figure 1:
Effective use of the Clearblue Easy (A) and the Persona (B) fertility monitors requires three steps: 1. A button marked "M" is pressed at the onset of menstruation. 2. Daily testing is performed by wetting a test stick with first morning urine and inserting the stick into the monitor. "High" and "peak" readings on the Clearblue Easy monitor, or a red light on the Persona monitor, indicate the fertile period. 3. With the Clearblue Easy monitor, testing starts on day 6 and continues until the last "high" reading (usually 10–15 days per month); with the Persona monitor, testing starts on day 6 and continues for 16 days in the first cycle and for 8 days in each subsequent cycle.
Because the Clearblue Easy fertility monitor is readily available in North America and the Persona monitor has not yet been approved by the US Food and Drug Administration or Health Canada, the Clearblue Easy monitor has been more widely adopted in Canada and the United States.
Who may benefit from using these devices?
Based on our clinical experience and that of others,10 fertility monitors have been well received by women from a variety of backgrounds, but these devices appear particularly well suited to selected niches. For example, women with conditions such as a history of migraines, deep vein thrombosis, pulmonary embolus or stroke, may find fertility monitoring to be a welcome alternative to hormonal contraception. Women concerned about environmental issues and escalating levels of synthetic estrogens excreted into the water supply4 may also wish to avoid taking hormones by using these devices as an alternative to oral contraceptives. Furthermore, women disinclined toward artificial methods of contraception for philosophical or religious reasons may find fertility monitoring to be consistent with their beliefs.
What is the evidence so far?
When used correctly, the Persona fertility monitor has an effectiveness rate of 93.8% for avoiding pregnancy.5 In addition, an Italian study showed that the fertile phase determined by the Persona monitor corresponded well with follicle growth and ovulation, as observed on ultrasound images and confirmed with serum luteinizing hormone and progesterone levels.6 In 94% of cycles, the first day of the potential fertile window (determined by ultrasonography) corresponded to the first instance of a red light displayed on the monitor; in 95.8% of cycles, ovulatory changes observed on ultrasound images occurred within the red-light period displayed on the monitor; and in 97.5% of cycles, the first day of infertility after ovulation observed on ultrasound images corresponded to the first post-ovulatory green light on the monitor.6
Similarly, a single-blinded prospective trial supported by the manufacturer correlated the readings on the Clearblue Easy fertility monitor with surges in serum luteinizing hormone levels and ovulation, as detected by transvaginal ultrasonography.7 The correlation showed that, 97% of the time, ovulation occurred within the two days of "peak" fertility plus one subsequent day of "high" fertility on the monitor, and no ovulations occurred before the monitor displayed a "peak" reading.7 In another study, urine levels of both estrone-3-glucuronide and luteinizing hormone measured using the Clearblue Easy monitor coincided well with serum measurements in identifying the potentially fertile phase.8 The exact effectiveness rates of using the Clearblue Easy monitor on its own for contraception have not yet been quantified. Therefore, the instructions for preventing pregnancy described in the caption to Figure 1 are based on clinical experience, not on evidence from randomized controlled trials.
Although studies showing positive and negative predictive values of the Clearblue Easy monitor for estrone-3-glucuronide measurement in relation to the possibility of pregnancy are lacking, a randomized controlled trial is currently underway to determine the daily probability of pregnancy,9 which will provide the sensitivity and specificity for the onset of fertility. Positive and negative predictive values for the onset of fertility with the Persona monitor have been calculated using the sensitivity and specificity values from the Italian study.6 The Persona monitor has a positive predictive value of 95.9% and a negative predictive value of 94.1%. Table 1 summarizes the sensitivity and specificity of the two fertility monitors.
Table 1:
Sensitivity and specificity of the Clearblue Easy and Persona fertility monitors in identifying the fertile phase
Although the predictive values for the onset of fertility using the Clearblue Easy fertility monitor have not yet been firmly established, as they have with the Persona monitor, a method of evaluating its effectiveness has been described in the literature. The "Marquette method" involves using the Clearblue Easy monitor in conjunction with observations of pre-ovulatory cervical mucus. An independent 12-month retrospective trial of this method involving 204 women revealed an effectiveness rate of 90.8% (95% confidence interval [CI] 83%–98%) with typical use and 99% (95% CI 90%–100%) with correct use.10 A satisfaction questionnaire in this study showed good overall satisfaction with the method; the mean response was 3.0 on a four-point scale, where 1 was "not satisfied," 2 was "unsure," 3 was "satisfied" and 4 was "very satisfied." A study to evaluate discontinuation rates with the Marquette method showed that 1.5% stopped for health reasons, 9.1% stopped for personal reasons, 9.7% stopped to pursue pregnancy and 15.4% were lost to follow-up.11
In a more recent retrospective cohort study comparing family planning techniques, the Marquette method was associated with a pregnancy rate of 2.0% with correct use and 12% with typical use; it was shown to be more effective than observation of cervical mucus alone.12 Most of the participants in these trials, however, are white married couples in their late twenties; as such, the effectiveness rate with typical use of the device may not be applicable to all populations. Independent, prospective randomized controlled trials are underway to replicate these outcomes.
What are the possible harms?
Although safety and freedom from adverse effects are strong features of fertility monitors, they have limitations, as does any innovation (Table 2). Just as oral contraceptives require regular intake to be effective, fertility monitors require regular urine sampling on 10–15 days of a woman's cycle. If couples choose to have intercourse on "high" or "peak" days using the Clearblue Easy fertility monitor, or "red" days using the Persona monitor, alternate protection may be required to avoid unintended pregnancy.
Table 2:
Pros and cons of using a personal fertility monitor for family planning
The Clearblue Easy fertility monitor may be less reliable in women with short fertile phases. These women would benefit from the longer window defined by the Persona monitor, a double-check method as provided by the Marquette method or additional forms of protection at the onset of the fertile phase.
The financial outlay involves the cost of acquiring the monitor and the cost of purchasing monthly test strips. Fertility monitors are not yet covered on any Canadian formularies, but they may be eligible for coverage under private health insurance plans.
As with other nonbarrier methods of contraception, no protection against sexually transmitted infections is offered when using the monitors on their own.
What can we expect in the future?
Modern couples now have the opportunity to consider various options for safe and effective family planning to suit a plurality of backgrounds, beliefs and aspirations. As user-friendly devices that require little instruction, the Clear-Blue Easy and Persona fertility monitors offer innovative approaches to contraception. Although extensive efficacy studies on these devices are not yet available, the preliminary evidence of effectiveness is promising.
Given the risks associated with some forms of contraception and the emerging recognition of the potential impact of hormonal agents on the environment, fertility monitors may find a welcome place in the current array of contraceptive options. Although additional studies on efficacy are needed, these monitors appear to be well received, especially by women who are not able to, or choose not to, use other forms of family planning. Fertility monitors can be used without the need for extensive training, new skills or knowledge for prescribing clinicians. In addition, industry-produced materials are available on the use of these user-friendly devices, and minimal time or effort is required for instruction by health providers. For these reasons, fertility monitoring, as predicted by Dr. Djerassi, is expected to become increasingly popular as a method of reliable and safe family planning.
-
Hand-held devices that measure urine levels of estrone-3-glucuronide and luteinizing hormone can be used at home to determine the fertile period.
-
Women may use these devices to prevent pregnancy by avoiding intercourse (or using other precautions) when increases in the hormone levels are detected.
-
The Persona monitor has an effectiveness rate of 93.8% with correct use for avoiding pregnancy, and the Clearblue Easy monitor, in conjunction with observation of ovulatory mucous, has a correct-use effectiveness rate of 99%.
-
Although further study is required, the use of personal fertility monitors may be a useful option for family planning.
Key points
Footnotes
-
This article has been peer reviewed.
-
Competing interests: None declared.
-
Contributors: Both authors contributed substantially to the writing and revision of the manuscript for important intellectual content and approved the final version submitted for publication.
References
- ↵
- ↵
World Health Organization, Task Force on Methods for the Determination of the Fertile Period, Special Programme of Research, Development and Research Training in Human Reproduction. Temporal relationships between ovulation and defined changes in the concentration of plasma estradiol-17 beta, luteinizing hormone, follicle-stimulating hormone, and progesterone. I. Probit analysis. Am J Obstet Gynecol 1980;138:383–90.
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
Source: https://www.cmaj.ca/content/183/1/73
0 Response to "Using Clear Blue Easy Fertility Monitor to Avoid"
Post a Comment